|
OZONE
Pleas use our A-Z INDEX to navigate this site
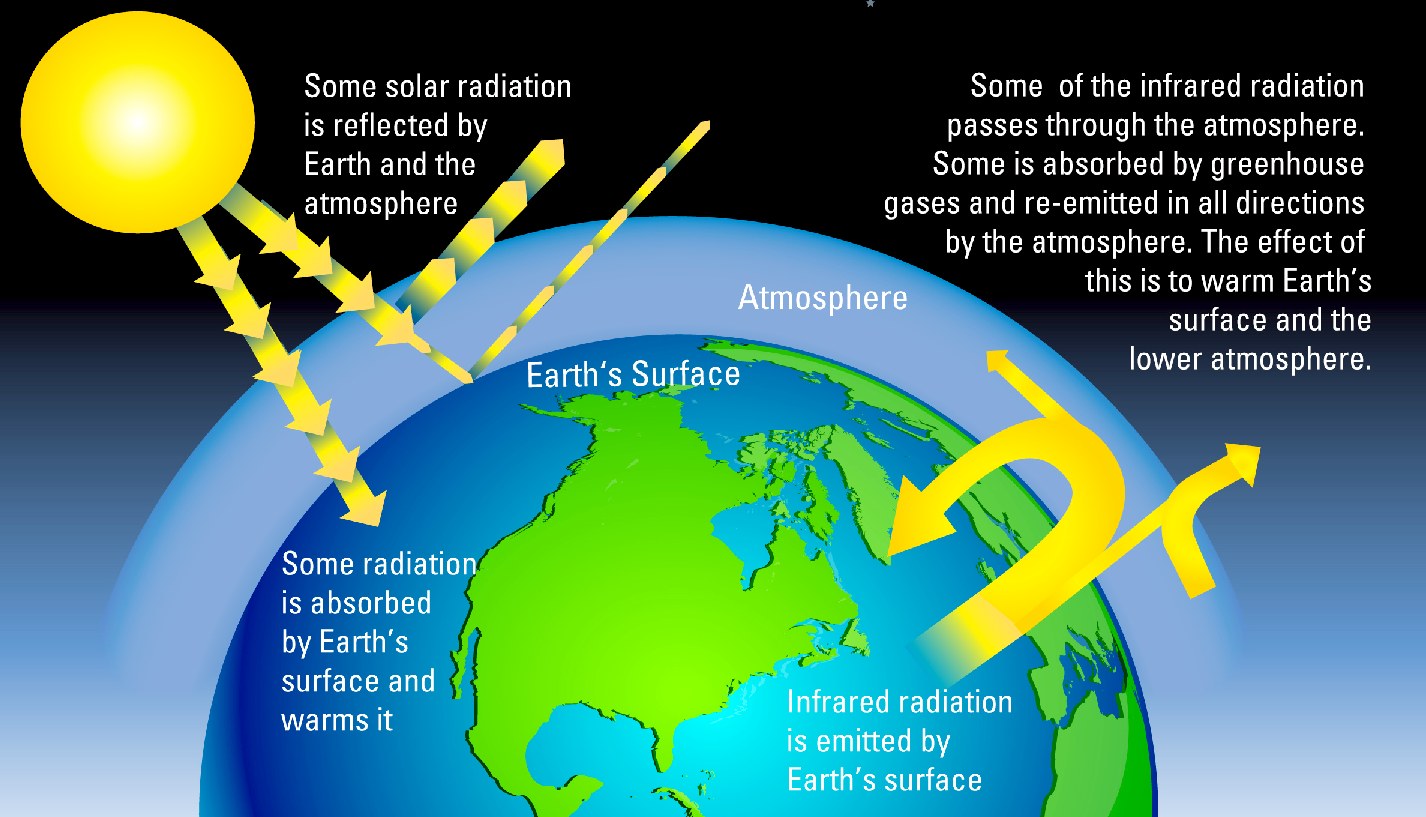
JUST LIKE A GLASS BOWL - Radiation from the sun would be reflected out into space, but with more greenhouse gases in the atmosphere some radiated heat is absorbed and re-emitted back to earth just as if we lived in a greenhouse.
Ozone was present at ground level before the Industrial Revolution, peak concentrations are now far higher than the pre-industrial levels, and even background concentrations well away from sources of pollution are substantially higher. Ozone acts as a greenhouse gas, absorbing some of the infrared energy emitted by the earth. Quantifying the greenhouse gas potency of ozone is difficult because it is not present in uniform concentrations across the globe. However, the most widely accepted scientific assessments relating to climate change (e.g. the Intergovernmental Panel on Climate Change Third Assessment Report) suggest that the radiative forcing of tropospheric ozone is about 25% that of carbon dioxide.
This means that over a 20-year span, the global warming potential of tropospheric ozone is much less, roughly 62 to 69 tons carbon dioxide equivalent / ton tropospheric ozone.
By far the biggest contributor to global warming after water vapour, is Carbon Dioxide. We cannot do much about water vapour where around 71% of the Earth's surface is water. But, the rate of evaporation is dependent on temperature, hence the other gases that contribute to the glass-like bubble we live in are very important.
The major greenhouse gases are water vapour, which causes about 36–70% of the greenhouse effect; carbon dioxide (CO2), which causes 9–26%; methane (CH4), which causes 4–9%; nitrous oxide (N2O) that accounts for about 5.6 percent of greenhouse gas emissions from human activities and ozone (O3), which causes 3–7%.
Ozone in the ozone layer filters out sunlight wavelengths from about 200 nm UV rays to 315 nm, with ozone peak absorption at about 250 nm. This ozone UV absorption is important to life, since it extends the absorption of UV by ordinary oxygen and nitrogen in air (which absorb all wavelengths < 200 nm) through the lower UV-C (200–280 nm) and the entire UV-B band (280–315 nm).
The small unabsorbed part that remains of UV-B after passage through ozone causes sunburn in humans, and direct
DNA damage in living tissues in both plants and animals. Ozone's effect on mid-range UV-B rays is illustrated by its effect on UV-B at 290 nm, which has a radiation intensity 350 million times as powerful at the top of the atmosphere as at the surface. Nevertheless, enough of UV-B radiation at similar frequency reaches the ground to cause some sunburn, and these same wavelengths are also among those responsible for the production of vitamin D in humans.
Ozone has been shown to affect the respiratory, cardiovascular and central nervous system. Early death and problems in reproductive health and development are also shown to be associated with ozone exposure.
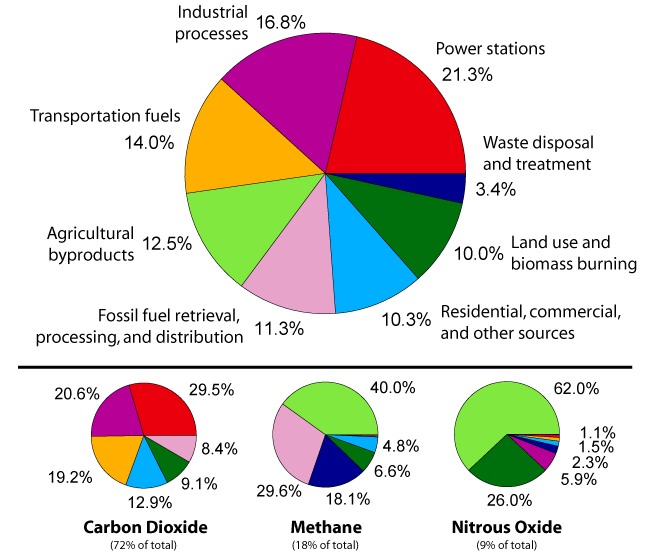
THE CAUSES IN SECTORS - Transportation and energy for living are the main causes of greenhouse gas build up in the atmosphere as carbon dioxide and nitrous oxides, with agriculture loading us with methane and more nitrous oxides. Hence, we need to revise our eating habits and switch to renewable energy for our cars and industry as quickly as possible.
AIR POLLUTION
Ozone precursors are a group of pollutants, predominantly those emitted during the combustion of fossil fuels. Ground-level ozone pollution (tropospheric ozone) is created near the Earth's surface by the action of daylight UV rays on these precursors. The ozone at ground level is primarily from fossil fuel precursors, but methane is a natural precursor, and the very low natural background level of ozone at ground level is considered safe.
GREENHOUSE GASES
The greenhouse effect is the process by which absorption and emission of infrared radiation by gases in a planet's atmosphere, warm its lower atmosphere and surface, to include warming the oceans and melting the ice caps.
LINKS & REFERENCE
https://www
Pleas use our A-Z INDEX to navigate this site
This website is provided on a free basis as a public information service. copyright © Climate Change Trust 2019. Solar Studios, BN271RF, United Kingdom.
|